Theme: Integrating precision in methodology, design and analysis of advanced clinical trials
Clinical Trials Congress 2019
6th World Congress on Advanced Clinical Trials and Clinical Research, (Clinical Trials Congress 2019) scheduled to be held during November 19-20, 2019 at Frankfurt, Germany. This Clinical Trials Congress hosts a wide range of Keynote presentations, Oral talks, Poster presentations, Symposia, Workshops, Exhibitions, and Career development programs. It strives to bring all the Researchers, Students and Business people under one roof to share their ideas and conduct collaborated research.
Why Attend to Clinical Trials Congress 2019?
Clinical Trials Congress 2019 Conference is a multidisciplinary program with broad participation with members from around the globe focused on learning about clinical research and its advances. The program focuses on key issues and opportunities in the clinical trial industry, including patient recruitment, site selection, data integration, existing data sources, mobile tech, project management, outsourcing, vendor management, budgeting and contracting, quality (QbD) in trial conduct, risk-based monitoring, Bioinformatics in clinical research and trials, Nanotechnology in clinical trials, Entrepreneurs investment meet, Postmarketing surveillance and clinical auditing. This is your best opportunity to reach the largest assemblage of participants from Clinical Trials Congress community which includes academia, clinical research entities, medical groups, related associations, societies, government agencies, pharmaceutical, biomedical device industries, and many business groups.
Clinical Trials Congress 2019 will discuss various disciplines involved in the pre-clinical research, conduct of clinical trials; it will educate health care researchers about design, operation, organizing, research computing, regulatory aspects and reporting of clinical trials. It promotes better understanding by the general public about the importance of clinical trials in prevention, diagnosis, Ethics related to the clinical trials and treatment of disease. This conference conduct presentations, distribute information, meet with current and potential scientists, make a splash with new clinical research developments, and receive name recognition at this 2-days event. World-renowned speakers and the most recent techniques, developments, the newest updates in Clinical Research are hallmarks of this conference. And also we try to encourage the budding researchers by felicitating them with attractive prizes
Who should attend Clinical Trials Congress 2019 and Who You’ll Meet
Directors/Senior Directors/Executive Directors and Vice Presidents/Senior Vice Presidents/Executive Vice Presidents and Heads/Leaders/Partners of
- CROs and CMOs
- Clinical Research Sites
- Pharma/Biotech and Medical Device industries
- Hospitals, Associations
- Clinical research institutes
- Societies
Medical Directors, Principal Investigators, Methodologists, and other clinical research professionals along with Academicians: University Faculties like Directors, Senior Professors/Assistant Professors/ Associate Professor, Research Scholars, scientists who are related to clinical and medical research.
Clinical/Pharmaceutical and biotech industry professionals with responsibilities in:
- Clinical Research & Development
- Clinical Bioinformatics
- Clinical Nanotechnology
- Clinical Design/ Protocol Design/ Clinical Strategy
- Global Clinical Operations/ Clinical Outsourcing
- Biostatistics/Data management
- Patient Recruitment/Enrolment
- Clinical Trial Management/Clinical Trial Supplies
- Regulatory Affairs
Sessions/Tracks
Conference Series invites all the interested participants from all over the world to attend "6th World Congress on Advanced Clinical Trials and Clinical Research" scheduled to be held during November 19-20, 2019 at Frankfurt, Germany. Which will emphasize on the recent developments in the field of Clinical Trials and Clinical Research? This event will be a gathering of many renowned Speakers, Clinical Trials and Research practitioners, Educational Institutions, Pharma and Biotech Companies, Research Scholars and Students who will have interactive sessions on the following topics.
Track 1 : Clinical Research & Clinical Trials: Academic Perspective
Clinical trial is a part of clinical research that follows a regulated protocol, or plan of action. Clinical trials are primarily performed to get data on safety and efficacy of the new developed drug, this data is mandatory for further approval of the drug and to bring it into the market.
The clinical trials market has been estimated to reach USD 14.2 billion in 2016 and is projected to reach around USD 22 billion by the year 2021, growing at a CAGR (compounded annual growth rate) of 7.5%, during the forecast period 2016 to 2021. Key drivers impacting the market growth are globalization of clinical trials, development of new treatments such as personalized medicine, augmenting evolution in technology, and boosting demand for CROs to conduct clinical trials. Clinical studies can be sponsored, or funded, by pharmaceutical companies, academic medical centers, voluntary groups, and other organizations, in addition to Federal agencies such as the National Institutes of Health, the U.S. Department of Defense, and the U.S. Department of Veterans Affairs. Doctors, other health care providers, and other individuals can also sponsor clinical research.
Clinical Trials Conferences | Clinical Research Conferences | Drug Discovery and Development Conferences
Relevant Conferences:
Basket and Umbrella Trials for Clinical Oncology, October 17-18, 2018, Philadelphia, PA. 7th Annual Publication and Clinical Trial Transparency, June 20-21, 2018, Amsterdam, The Netherlands. Direct-to-Patient Clinical Trials, August 9-10, 2018, Philadelphia, PA. 7th International Conference on Clinical Trials, September 24-26, 2018, Chicago, Illinois, USA.
Track 2 : Clinical Data Management
Clinical data management is the process of collection, cleaning, integration and management of subject data in compliance with regulatory standards. It is a critical phase in clinical research, which leads to generation of high-quality, reliable, and statistically sound data from clinical trials, this has been facilitated by the use of software applications that maintain an audit trail and provide easy identification and resolution of data discrepancies. CDM also supports the conduct, management and analysis of studies across the spectrum of clinical research. The ultimate goal of CDM is to assure that data support conclusions drawn from research. Achieving this goal protects public health and confidence in marketed therapeutics.
Clinical Trials Societies | Clinical Research Societies | Clinical Trials and Research Associations
Relevant Conferences:
Basket and Umbrella Trials for Clinical Oncology, October 17-18, 2018, Philadelphia, PA. 7th Annual Publication and Clinical Trial Transparency, June 20-21, 2018, Amsterdam, The Netherlands. Direct-to-Patient Clinical Trials, August 9-10, 2018, Philadelphia, PA. 7th International Conference on Clinical Trials, September 24-26, 2018, Chicago, Illinois, USA.
Track 3 :Clinical and Medical Case Reports
A case report is a means of communicating something new that has been learnt from clinical practice. It could be about an unusual or previously unknown condition, a rare presentation or complication of a known disease, or even a new approach to managing a common condition. A case report provides the detailed report of symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports may contain a demographic profile of the patient and play major role in the field of medical research and evidenced based medicine. Moreover, case reports can serve as an early warning signal for the adverse effects of new medications, or the presentations of new and emerging diseases
Clinical Trials Events | Clinical Trials Conferences | Clinical Trials Meetings
Relevant Conferences:
International Conference On Clinical Trials and Clinical Research, Toronto, Canada, September 24-26, 2018. Clinical Trial Supply Chain Strategy Meeting Europe 2018, 27 Sep 2018, Switzerland. Clinical Operations Strategy Meeting APAC 2018, 09 Oct 2018, Singapore. SCOE — Summit for Clinical Trials Operations Executives Europe, Oct 2018 Barcelona, Spain.
Track 4 :Nano Technology in Clinical Trials
Nanotechnology ("nanotech") is the manipulation of matter on an atomic, molecular, and supra molecular scale. Nanotechnology as defined by size is naturally very broad, including fields of science as diverse as surface science, organic chemistry, molecular biology, semiconductor physics, micro fabrication, etc. The associated research and applications are equally diverse, ranging from extensions of conventional device physics to completely new approaches based upon molecular self-assembly, from developing new materials with dimensions on the nano scale to direct control of matter on the atomic scale.
NanoTech Conferences | Clinical Research Events | Clinical Trials Events
Relevant Conferences:
Basket and Umbrella Trials for Clinical Oncology, October 17-18, 2018, Philadelphia, PA. 7th Annual Publication and Clinical Trial Transparency, June 20-21, 2018, Amsterdam, The Netherlands. Direct-to-Patient Clinical Trials, August 9-10, 2018, Philadelphia, PA. 7th International Conference on Clinical Trials, September 24-26, 2018, Chicago, Illinois, USA.
Track 5 : Clinical Trials Conducts
Clinical Operations have a lot of interaction with people in a range of other departments including Clinical Science, Clinical Quality Assurance, Data Management, Biostatistics and Regulatory Affairs to ensure that the data and information needed by these other departments is delivered so they can decide if a trial has been successful. The Clinical Operations function of a company is key to the delivery of clinical trials. Without this team no Clinical Research activity could be delivered. Clinical Operations teams are responsible for designing, planning and physically running Phase I – IV clinical trials. Many larger pharmaceutical companies have also looked at setting up strategic partnerships with Clinical Research Organizations to outsource some or all of their Clinical Operations activities.
Maintain required records of study activity including case report forms, drug dispensation records, or regulatory forms. Assess eligibility of potential subjects through methods such as screening interviews, reviews of medical records, and discussions with physicians and nurses. Identify protocol problems, inform investigators of problems, or assist in problem resolution efforts such as protocol revisions.
Clinical Trials Conferences | Clinical Research Conferences | Drug Discovery and Development Conferences
Relevant Conferences:
International Conference On Clinical Trials and Clinical Research, Toronto, Canada, September 24-26, 2018. Clinical Trial Supply Chain Strategy Meeting Europe 2018, 27 Sep 2018, Switzerland. Clinical Operations Strategy Meeting APAC 2018, 09 Oct 2018, Singapore. SCOE — Summit for Clinical Trials Operations Executives Europe, Oct 2018 Barcelona, Spain
Track 6 :Drug Discovery and Development
Researchers discover new drugs through insights into a disease process that allow researchers to design a product to stop or reverse the effects of the disease. Once researchers identify a promising compound for development, they conduct experiments to gather information on how it is absorbed, distributed, metabolized, and excreted, best dosage, Side effects, how it interacts with other drugs and treatments and its effectiveness as compared with similar drugs.
Bringing one new drug to the public typically costs a pharmaceutical or biotechnology company on average more than $1 billion and takes an average of 10 to 15 years. Each drug undergoes a stringent process of discovery, development, approval and finally, public use
Clinical Trials Conferences | Clinical Research Conferences | Drug Discovery and Development Conferences
Relevant Conferences:
International Conference On Clinical Trials and Clinical Research, Toronto, Canada, September 24-26, 2018. Clinical Trial Supply Chain Strategy Meeting Europe 2018, 27 Sep 2018, Switzerland. Clinical Operations Strategy Meeting APAC 2018, 09 Oct 2018, Singapore. SCOE — Summit for Clinical Trials Operations Executives Europe, Oct 2018 Barcelona, Spain.
Track 7 :Pharmacogenomics
Pharmacogenetics refers to how variation in one single gene influences the response to a single drug and Pharmacogenomics refers to how all of the genes (the genome) can influence responses to drugs. The general feature of these diverse lesions is that two nucleotides on opposite strands are joined covalently. Mutagenicity and carcinogenicity are clearly correlated.Understanding the specificity of mutagens in bacteria has led to the direct implication of certain environmental mutagens in the causation of human cancers
The Pharmaceutical industry's long successful strategy of placing big bets on a few molecules, promoting them heavily and turning them into blockbusters worked well for many years, but its R&D productivity has now plummeted and the environment’s changing. Regulators are becoming more cautious about approving truly innovative medicines
Pharmacogenomics Conferences | Pharmacogenomics Meetings | Clinical Research Conferences
Relevant Conferences:
International Conference On Clinical Trials and Clinical Research, Toronto, Canada, September 24-26, 2018. Clinical Trial Supply Chain Strategy Meeting Europe 2018, 27 Sep 2018, Switzerland. Clinical Operations Strategy Meeting APAC 2018, 09 Oct 2018, Singapore. SCOE — Summit for Clinical Trials Operations Executives Europe, Oct 2018 Barcelona, Spain.
Track 8 :Globalization of Clinical Trials
The globalization of clinical research is a relatively recent phenomenon, in which many of these studies are taking place on a global scale, with a significant increase of clinical trials in developing countries. Developed markets in the United States, Western Europe, Germany, and Japan still generate the lion’s share of clinical trial activity. Nearly 31% of the world's clinical trials are reportedly conducted outside of the United. According to the report China, Japan, India, and Korea are the most active settings for clinical trials among developing nations. It is predicted that Japan as the world’s second-largest pharmaceutical market by 2015.
According to the ClinicalTrials.gov the total number of studies registered in 2016 is 231,756. The percentage of studies registered from United States is 37%, Non-U.S is 47%. It is estimated to reach more than 280,000 study registries by 2017.
Clinical Trials Societies | Clinical Research Societies | Clinical Trials and Research Associations
Relevant Conferences:
Basket and Umbrella Trials for Clinical Oncology, October 17-18, 2018, Philadelphia, PA. 7th Annual Publication and Clinical Trial Transparency, June 20-21, 2018, Amsterdam, The Netherlands. Direct-to-Patient Clinical Trials, August 9-10, 2018, Philadelphia, PA. 7th International Conference on Clinical Trials, September 24-26, 2018, Chicago, Illinois, USA.
Track 9 :Pharmacoepidemiology
Pharmacoepidemiology is the investigation of the way in which sub-atomic biomarkers change the clinical impacts of medicines in common people. Similarly as the essential exploration of Pharmacoepidemiology is the study of disease transmission, connected to the substance territory of clinical pharmacology, the fundamental art of atomic Pharmacoepidemiology is the study of disease transmission when all is said in done and sub-atomic the study of disease transmission particularly, likewise connected to the substance region of clinical pharmacology. Subsequently a considerable lot of strategies identified with the study of disease transmission are apply to sub-atomic Pharmacoepidemiology thinks about. Pharmacoepidemiology used to comprehend the intricate connection between medicine reaction and the immense number of potential sub-atomic and hereditary impacts on this reaction; an attention on cooperations among these elements and collaborations amongst qualities and condition.
Pharmacoepidemiology Conferences | Pharmacoepidemiology Meetings | Pharmacoepidemiology Events
Relevant Conferences:
Basket and Umbrella Trials for Clinical Oncology, October 17-18, 2018, Philadelphia, PA. 7th Annual Publication and Clinical Trial Transparency, June 20-21, 2018, Amsterdam, The Netherlands. Direct-to-Patient Clinical Trials, August 9-10, 2018, Philadelphia, PA. 7th International Conference on Clinical Trials, September 24-26, 2018, Chicago, Illinois, USA.
Track 10 :Patient-Centric Clinical Trials
Generally accepted principles suggest that patient involvement should extend well beyond consideration as research subjects. Patients are key stakeholders in all aspects of trial design & execution. Patient-centric drug development also offers a huge opportunity to define meaningful outcomes from the patient perspective, as a way to ensure the needs and priorities of patient populations are reflected in research. Although efforts are made to control risks to clinical trial participants, some risk may be unavoidable because of the uncertainty inherent in clinical research involving new medical products. It's important, therefore, that people make their decision to participate in a clinical trial only after they have a full understanding of the entire process and the risks that may be involved.
Clinical Trials Conferences | Clinical Research Conferences | Clinical Reseach meetings
Relevant Conferences:
International Conference On Clinical Trials and Clinical Research, Toronto, Canada, September 24-26, 2018. Clinical Trial Supply Chain Strategy Meeting Europe 2018, 27 Sep 2018, Switzerland. Clinical Operations Strategy Meeting APAC 2018, 09 Oct 2018, Singapore. SCOE — Summit for Clinical Trials Operations Executives Europe, Oct 2018 Barcelona, Spain.
Track 11 :Research and Trials on Oncology and AIDS
HIV clinical trials are research studies that look at new ways to prevent, detect, or treat HIV/AIDS. Clinical trials are the fastest way to determine if new medical approaches to HIV/AIDS are safe and effective in people. clinical trials under way include studies of new HIV medicines, studies of vaccines to prevent or treat HIV, and studies of medicines to treat infections related to HIV.
There are several types of cancer clinical trials, including treatment trials, prevention trials, screening trials, and supportive and palliative care trials. Each type of trial is designed to answer different research questions and will help researchers learn things that will help people in the future.
Clinical Trials Societies | Clinical Research Societies | Clinical Trials and Research Associations
Relevant Conferences:
Basket and Umbrella Trials for Clinical Oncology, October 17-18, 2018, Philadelphia, PA. 7th Annual Publication and Clinical Trial Transparency, June 20-21, 2018, Amsterdam, The Netherlands. Direct-to-Patient Clinical Trials, August 9-10, 2018, Philadelphia, PA. 7th International Conference on Clinical Trials, September 24-26, 2018, Chicago, Illinois, USA.
Track 12 :Pharmacovigilance and Drug Safety
The pharmacovigilance is related to collection, detection, assessment, monitoring, and prevention of adverse effects with pharmaceutical products, and it is needed in different stages of product life cycle, and the safety surveillance and risk management. Information received from patients and healthcare providers via pharmacovigilance agreements, plays a critical role in providing the data necessary for Pharmacovigilance to take place, in order to market or to test a pharmaceutical product, adverse event data must be submitted to the local drug regulatory authority. Finally pharmacovigilance is concerned with identifying the hazards associated with pharmaceutical products and with minimizing the risk of any harm that may come to patients by safety surveillance and risk management
Clinical Trials Societies | Clinical Research Societies | Clinical Trials and Research Associations
Relevant Conferences:
Basket and Umbrella Trials for Clinical Oncology, October 17-18, 2018, Philadelphia, PA. 7th Annual Publication and Clinical Trial Transparency, June 20-21, 2018, Amsterdam, The Netherlands. Direct-to-Patient Clinical Trials, August 9-10, 2018, Philadelphia, PA. 7th International Conference on Clinical Trials, September 24-26, 2018, Chicago, Illinois, USA.
Track 13 :Bioinformatics in Clinical Research
Bioinformatics in Clinical Research the typically controversial ethical issues emerging from new situations and possibilities brought about by advances in medicine. It is also moral discernment as it relates to medical policy, practice, and research. Bioethicists are concerned with the ethical questions that arise in the relationships among life sciences, biotechnology, medicine, clinical research, and philosophy etc. One of the first areas addressed by modern bioethicists was that of human experimentation. The National Commission for the Protection of Human Subjects of Biomedical and Behavioural Research was initially established in 1974 to identify the basic ethical principles that should underlie the conduct of biomedical and behavioral research involving human subjects.
Clinical research ethics are the set of relevant ethics considered in the conduct of a clinical trial in the field of clinical research. It borrows from the broader fields of research ethics and medical ethics. Quality of clinical trials depends on data integrity and subject protection. Good Clinical Practice (GCP) is the universal ethical and scientific quality standard for conducting clinical trials. The GCP standard applies to all aspects of the clinical trial process.
Bioinformatics Conferences | Biostatistics Conferences | Clinical Trials Conferences
Relevant Conferences:
International Conference On Clinical Trials and Clinical Research, Toronto, Canada, September 24-26, 2018. Clinical Trial Supply Chain Strategy Meeting Europe 2018, 27 Sep 2018, Switzerland. Clinical Operations Strategy Meeting APAC 2018, 09 Oct 2018, Singapore. SCOE — Summit for Clinical Trials Operations Executives Europe, Oct 2018 Barcelona, Spain.
Track 14 :Clinical Trials on Different Diseases
Clinical Trials for different diseases and disorders are conducted for evaluating one or more interventions (for example, drugs, medical devices, approaches to surgery or radiation therapy) for treating a disease, syndrome, or condition and also finding ways to prevent the initial development or recurrence of a disease or condition. These can include medicines, vaccines, or lifestyle changes, among other approaches. Some examples of the diseases/disorders for which clinical trials conducting are Cardiovascular, Digestive system, Respiratory system diseases and other parasitic, viral, bacterial and fungal diseases. And Clinical Trials on behaviors, mental, sleep and eating disorders.
Rare Disease Conferences | Medicine Conferences | Medical Research Conferences
Relevant Conferences:
International Conference On Clinical Trials and Clinical Research, Toronto, Canada, September 24-26, 2018. Clinical Trial Supply Chain Strategy Meeting Europe 2018, 27 Sep 2018, Switzerland. Clinical Operations Strategy Meeting APAC 2018, 09 Oct 2018, Singapore. SCOE — Summit for Clinical Trials Operations Executives Europe, Oct 2018 Barcelona, Spain.
Track 15 :CRO (Contract Research Organization) Clinical Trials
CRO (Contract Research Organization) is an organization that provides support to the pharmaceutical, biotechnology, and medical device industries in the form of research services outsourced on a contract basis. Many CROs specifically provide clinical-study and clinical-trial support for drugs and/or medical devices. CROs range from large, international full-service organizations to small, niche specialty groups. A CRO may provide such services as biopharmaceutical development, biologic assay development, commercialization, preclinical research, clinical research, clinical trials management, and pharmacovigilance. CROs also support foundations, research institutions, and universities, in addition to government organizations.
Sponsorship: In the conduct of clinical trials, a sponsor is an individual (institution, company or organization) that takes the responsibility to initiate, manage or finance the clinical trial, but does not actually conduct the investigation. A sponsor-investigator, on the other hand, takes on the responsibility as a clinical study sponsor and also conducts or oversees the clinical trial. Thus, a sponsor-investigator must comply with the applicable regulatory requirements that pertain to both the sponsor and the investigator
Clinical Research Conferences | CRO Meetings | CRO Conferences
Relevant Conferences:
Basket and Umbrella Trials for Clinical Oncology, October 17-18, 2018, Philadelphia, PA. 7th Annual Publication and Clinical Trial Transparency, June 20-21, 2018, Amsterdam, The Netherlands. Direct-to-Patient Clinical Trials, August 9-10, 2018, Philadelphia, PA. 7th International Conference on Clinical Trials, September 24-26, 2018, Chicago, Illinois, USA.
Track 16 :Future Perspectives and Innovations in Clinical Studies
Clinical study design comprises the quantity of study volunteers, their segmentation based on varying factors, and their treatment throughout the clinical trial process. Study design is a key component of clinical trials, and the treatment of all patients directly impacts the statistical validity of data. Study group assignment has also been comprehensively improved in recent years. Researchers have found many benefits to randomized assignment versus observational assignment, based on characteristics like gender, age, race, etc. The randomized method has been found to yield more reliable results than observational study assignments.
In recent years, the use of Adaptive design methods in clinical research has become increasingly popular due to its flexibility and efficiency. Adaptive designs offer the potential to reduce study duration and patient exposure whilst maximizing the probability of a successful outcome. Another innovation in clinical trials is the Bucket design. Bucket trials are designed to utilize one particular drug and test that drug against a number of different diseases. The advantage of this approach is that patients with different diseases can be 'pooled' together under one larger trial instead of lots of smaller trials, thereby saving time and resource in a similar approach, and there are more innovations in clinical trials.
Clinical Trials Conferences | Clinical Research Conferences | Clinical Research Meetings
Relevant Conferences:
Basket and Umbrella Trials for Clinical Oncology, October 17-18, 2018, Philadelphia, PA. 7th Annual Publication and Clinical Trial Transparency, June 20-21, 2018, Amsterdam, The Netherlands. Direct-to-Patient Clinical Trials, August 9-10, 2018, Philadelphia, PA. 7th International Conference on Clinical Trials, September 24-26, 2018, Chicago, Illinois, USA.
Track 17 :In Silico Clinical Trials
Generally accepted principles suggest that patient involvement should extend well beyond consideration as research subjects. Patients are key stakeholders in all aspects of trial design & execution. Patient-centric drug development also offers a huge opportunity to define meaningful outcomes from the patient perspective, as a way to ensure the needs and priorities of patient populations are reflected in research. Although efforts are made to control risks to clinical trial participants, some risk may be unavoidable because of the uncertainty inherent in clinical research involving new medical products. It's important, therefore, that people make their decision to participate in a clinical trial only after they have a full understanding of the entire process and the risks that may be involved.
Clinical Trials Conferences | Clinical Research Conferences | Healthcare Conferences
Relevant Conferences:
International Conference On Clinical Trials and Clinical Research, Toronto, Canada, September 24-26, 2018. Clinical Trial Supply Chain Strategy Meeting Europe 2018, 27 Sep 2018, Switzerland. Clinical Operations Strategy Meeting APAC 2018, 09 Oct 2018, Singapore. SCOE — Summit for Clinical Trials Operations Executives Europe, Oct 2018 Barcelona, Spain.
Track 18 : Outsourcing and Collaborative Research in Clinical Trials
Outsourcing and Collaborative Research in Clinical Trialsbrings together CROs, sites, sponsors and vendors to collaborate on best practices for ensuring accurate and effective budgeting and forecasting in clinical trials. With growing complexities and shrinking tolerance for variance between forecasted and actual budget, it’s critical that internal and external teams work together to tackle the challenges and establish efficient processes. Join your trial counterparts in May to bridge the gap between finance and operations and address each player’s role in managing deviations including timeline delays, changes in study design, outsourcing and more.
Clinical Outsourcing | Clinical Research Conferences | Clinical Trials Conferences
Relevant Conferences:
Basket and Umbrella Trials for Clinical Oncology, October 17-18, 2018, Philadelphia, PA. 7th Annual Publication and Clinical Trial Transparency, June 20-21, 2018, Amsterdam, The Netherlands. Direct-to-Patient Clinical Trials, August 9-10, 2018, Philadelphia, PA. 7th International Conference on Clinical Trials, September 24-26, 2018, Chicago, Illinois, USA.
Track 19 :Gastrointestinal-oncology
Gastrointestinal oncology refers to malignant conditions of the gastrointestinal tract (GI tract) and accessory organs of digestion, including the oesophagus, stomach, biliary system, pancreas, small and large intestine, rectum and anus. The symptoms relate to the organ affected and can include obstruction which leads to difficulty swallowing or defecating, abnormal bleeding. The treatment requires endoscopy, followed by biopsy of suspicious tissue. The treatment depends on the location of the tumor, as well as the type of cancer cell and whether it has invaded other tissues or spread elsewhere. These factors also determine the prognosis.
Bioethics Conferences | Ethics Conferences | Clinical Trials Ethics
Relevant Conferences:
International Conference On Clinical Trials and Clinical Research, Toronto, Canada, September 24-26, 2018. Clinical Trial Supply Chain Strategy Meeting Europe 2018, 27 Sep 2018, Switzerland. Clinical Operations Strategy Meeting APAC 2018, 09 Oct 2018, Singapore. SCOE — Summit for Clinical Trials Operations Executives Europe, Oct 2018 Barcelona, Spain.
Track 20 :Entrepreneurs Investment Meet
Since the Designing and development of a pharmacological product is a process, single organizations cannot take up the cost of research involved in it. Entrepreneurs willing to invest in the Pharma sectors can meet together and have an idea about the Pharma product, it’s effectiveness, it’s market potential and scope. Since the Pharma sector is booming in the recent market scenario, investors can have a great share of profits.
Clinical Trials Societies | Clinical Research Societies | Clinical Trials and Research Associations
Relevant Conferences:
International Conference On Clinical Trials and Clinical Research, Toronto, Canada, September 24-26, 2018. Clinical Trial Supply Chain Strategy Meeting Europe 2018, 27 Sep 2018, Switzerland. Clinical Operations Strategy Meeting APAC 2018, 09 Oct 2018, Singapore. SCOE — Summit for Clinical Trials Operations Executives Europe, Oct 2018 Barcelona, Spain.
Track 1 : Clinical Research & Clinical Trials: Academic Perspective
Clinical trial is a part of clinical research that follows a regulated protocol, or plan of action. Clinical trials are primarily performed to get data on safety and efficacy of the new developed drug, this data is mandatory for further approval of the drug and to bring it into the market.
The clinical trials market has been estimated to reach USD 14.2 billion in 2016 and is projected to reach around USD 22 billion by the year 2021, growing at a CAGR (compounded annual growth rate) of 7.5%, during the forecast period 2016 to 2021. Key drivers impacting the market growth are globalization of clinical trials, development of new treatments such as personalized medicine, augmenting evolution in technology, and boosting demand for CROs to conduct clinical trials. Clinical studies can be sponsored, or funded, by pharmaceutical companies, academic medical centers, voluntary groups, and other organizations, in addition to Federal agencies such as the National Institutes of Health, the U.S. Department of Defense, and the U.S. Department of Veterans Affairs. Doctors, other health care providers, and other individuals can also sponsor clinical research.
Clinical Trials Conferences | Clinical Research Conferences | Drug Discovery and Development Conferences
Relevant Conferences:
Basket and Umbrella Trials for Clinical Oncology, October 17-18, 2018, Philadelphia, PA. 7th Annual Publication and Clinical Trial Transparency, June 20-21, 2018, Amsterdam, The Netherlands. Direct-to-Patient Clinical Trials, August 9-10, 2018, Philadelphia, PA. 7th International Conference on Clinical Trials, September 24-26, 2018, Chicago, Illinois, USA.
Track 2 : Clinical Data Management
Clinical data management is the process of collection, cleaning, integration and management of subject data in compliance with regulatory standards. It is a critical phase in clinical research, which leads to generation of high-quality, reliable, and statistically sound data from clinical trials, this has been facilitated by the use of software applications that maintain an audit trail and provide easy identification and resolution of data discrepancies. CDM also supports the conduct, management and analysis of studies across the spectrum of clinical research. The ultimate goal of CDM is to assure that data support conclusions drawn from research. Achieving this goal protects public health and confidence in marketed therapeutics.
Clinical Trials Societies | Clinical Research Societies | Clinical Trials and Research Associations
Relevant Conferences:
Basket and Umbrella Trials for Clinical Oncology, October 17-18, 2018, Philadelphia, PA. 7th Annual Publication and Clinical Trial Transparency, June 20-21, 2018, Amsterdam, The Netherlands. Direct-to-Patient Clinical Trials, August 9-10, 2018, Philadelphia, PA. 7th International Conference on Clinical Trials, September 24-26, 2018, Chicago, Illinois, USA.
Track 3 :Clinical and Medical Case Reports
A case report is a means of communicating something new that has been learnt from clinical practice. It could be about an unusual or previously unknown condition, a rare presentation or complication of a known disease, or even a new approach to managing a common condition. A case report provides the detailed report of symptoms, signs, diagnosis, treatment, and follow-up of an individual patient. Case reports may contain a demographic profile of the patient and play major role in the field of medical research and evidenced based medicine. Moreover, case reports can serve as an early warning signal for the adverse effects of new medications, or the presentations of new and emerging diseases
Clinical Trials Events | Clinical Trials Conferences | Clinical Trials Meetings
Relevant Conferences:
International Conference On Clinical Trials and Clinical Research, Toronto, Canada, September 24-26, 2018. Clinical Trial Supply Chain Strategy Meeting Europe 2018, 27 Sep 2018, Switzerland. Clinical Operations Strategy Meeting APAC 2018, 09 Oct 2018, Singapore. SCOE — Summit for Clinical Trials Operations Executives Europe, Oct 2018 Barcelona, Spain.
Track 4 :Nano Technology in Clinical Trials
Nanotechnology ("nanotech") is the manipulation of matter on an atomic, molecular, and supra molecular scale. Nanotechnology as defined by size is naturally very broad, including fields of science as diverse as surface science, organic chemistry, molecular biology, semiconductor physics, micro fabrication, etc. The associated research and applications are equally diverse, ranging from extensions of conventional device physics to completely new approaches based upon molecular self-assembly, from developing new materials with dimensions on the nano scale to direct control of matter on the atomic scale.
NanoTech Conferences | Clinical Research Events | Clinical Trials Events
Relevant Conferences:
Basket and Umbrella Trials for Clinical Oncology, October 17-18, 2018, Philadelphia, PA. 7th Annual Publication and Clinical Trial Transparency, June 20-21, 2018, Amsterdam, The Netherlands. Direct-to-Patient Clinical Trials, August 9-10, 2018, Philadelphia, PA. 7th International Conference on Clinical Trials, September 24-26, 2018, Chicago, Illinois, USA.
Track 5 : Clinical Trials Conducts
Clinical Operations have a lot of interaction with people in a range of other departments including Clinical Science, Clinical Quality Assurance, Data Management, Biostatistics and Regulatory Affairs to ensure that the data and information needed by these other departments is delivered so they can decide if a trial has been successful. The Clinical Operations function of a company is key to the delivery of clinical trials. Without this team no Clinical Research activity could be delivered. Clinical Operations teams are responsible for designing, planning and physically running Phase I – IV clinical trials. Many larger pharmaceutical companies have also looked at setting up strategic partnerships with Clinical Research Organizations to outsource some or all of their Clinical Operations activities.
Maintain required records of study activity including case report forms, drug dispensation records, or regulatory forms. Assess eligibility of potential subjects through methods such as screening interviews, reviews of medical records, and discussions with physicians and nurses. Identify protocol problems, inform investigators of problems, or assist in problem resolution efforts such as protocol revisions.
Clinical Trials Conferences | Clinical Research Conferences | Drug Discovery and Development Conferences
Relevant Conferences:
International Conference On Clinical Trials and Clinical Research, Toronto, Canada, September 24-26, 2018. Clinical Trial Supply Chain Strategy Meeting Europe 2018, 27 Sep 2018, Switzerland. Clinical Operations Strategy Meeting APAC 2018, 09 Oct 2018, Singapore. SCOE — Summit for Clinical Trials Operations Executives Europe, Oct 2018 Barcelona, Spain
Track 6 :Drug Discovery and Development
Researchers discover new drugs through insights into a disease process that allow researchers to design a product to stop or reverse the effects of the disease. Once researchers identify a promising compound for development, they conduct experiments to gather information on how it is absorbed, distributed, metabolized, and excreted, best dosage, Side effects, how it interacts with other drugs and treatments and its effectiveness as compared with similar drugs.
Bringing one new drug to the public typically costs a pharmaceutical or biotechnology company on average more than $1 billion and takes an average of 10 to 15 years. Each drug undergoes a stringent process of discovery, development, approval and finally, public use
Clinical Trials Conferences | Clinical Research Conferences | Drug Discovery and Development Conferences
Relevant Conferences:
International Conference On Clinical Trials and Clinical Research, Toronto, Canada, September 24-26, 2018. Clinical Trial Supply Chain Strategy Meeting Europe 2018, 27 Sep 2018, Switzerland. Clinical Operations Strategy Meeting APAC 2018, 09 Oct 2018, Singapore. SCOE — Summit for Clinical Trials Operations Executives Europe, Oct 2018 Barcelona, Spain.
Track 7 :Pharmacogenomics
Pharmacogenetics refers to how variation in one single gene influences the response to a single drug and Pharmacogenomics refers to how all of the genes (the genome) can influence responses to drugs. The general feature of these diverse lesions is that two nucleotides on opposite strands are joined covalently. Mutagenicity and carcinogenicity are clearly correlated.Understanding the specificity of mutagens in bacteria has led to the direct implication of certain environmental mutagens in the causation of human cancers
The Pharmaceutical industry's long successful strategy of placing big bets on a few molecules, promoting them heavily and turning them into blockbusters worked well for many years, but its R&D productivity has now plummeted and the environment’s changing. Regulators are becoming more cautious about approving truly innovative medicines
Pharmacogenomics Conferences | Pharmacogenomics Meetings | Clinical Research Conferences
Relevant Conferences:
International Conference On Clinical Trials and Clinical Research, Toronto, Canada, September 24-26, 2018. Clinical Trial Supply Chain Strategy Meeting Europe 2018, 27 Sep 2018, Switzerland. Clinical Operations Strategy Meeting APAC 2018, 09 Oct 2018, Singapore. SCOE — Summit for Clinical Trials Operations Executives Europe, Oct 2018 Barcelona, Spain.
Track 8 :Globalization of Clinical Trials
The globalization of clinical research is a relatively recent phenomenon, in which many of these studies are taking place on a global scale, with a significant increase of clinical trials in developing countries. Developed markets in the United States, Western Europe, Germany, and Japan still generate the lion’s share of clinical trial activity. Nearly 31% of the world's clinical trials are reportedly conducted outside of the United. According to the report China, Japan, India, and Korea are the most active settings for clinical trials among developing nations. It is predicted that Japan as the world’s second-largest pharmaceutical market by 2015.
According to the ClinicalTrials.gov the total number of studies registered in 2016 is 231,756. The percentage of studies registered from United States is 37%, Non-U.S is 47%. It is estimated to reach more than 280,000 study registries by 2017.
Clinical Trials Societies | Clinical Research Societies | Clinical Trials and Research Associations
Relevant Conferences:
Basket and Umbrella Trials for Clinical Oncology, October 17-18, 2018, Philadelphia, PA. 7th Annual Publication and Clinical Trial Transparency, June 20-21, 2018, Amsterdam, The Netherlands. Direct-to-Patient Clinical Trials, August 9-10, 2018, Philadelphia, PA. 7th International Conference on Clinical Trials, September 24-26, 2018, Chicago, Illinois, USA.
Track 9 :Pharmacoepidemiology
Pharmacoepidemiology is the investigation of the way in which sub-atomic biomarkers change the clinical impacts of medicines in common people. Similarly as the essential exploration of Pharmacoepidemiology is the study of disease transmission, connected to the substance territory of clinical pharmacology, the fundamental art of atomic Pharmacoepidemiology is the study of disease transmission when all is said in done and sub-atomic the study of disease transmission particularly, likewise connected to the substance region of clinical pharmacology. Subsequently a considerable lot of strategies identified with the study of disease transmission are apply to sub-atomic Pharmacoepidemiology thinks about. Pharmacoepidemiology used to comprehend the intricate connection between medicine reaction and the immense number of potential sub-atomic and hereditary impacts on this reaction; an attention on cooperations among these elements and collaborations amongst qualities and condition.
Pharmacoepidemiology Conferences | Pharmacoepidemiology Meetings | Pharmacoepidemiology Events
Relevant Conferences:
Basket and Umbrella Trials for Clinical Oncology, October 17-18, 2018, Philadelphia, PA. 7th Annual Publication and Clinical Trial Transparency, June 20-21, 2018, Amsterdam, The Netherlands. Direct-to-Patient Clinical Trials, August 9-10, 2018, Philadelphia, PA. 7th International Conference on Clinical Trials, September 24-26, 2018, Chicago, Illinois, USA.
Track 10 :Patient-Centric Clinical Trials
Generally accepted principles suggest that patient involvement should extend well beyond consideration as research subjects. Patients are key stakeholders in all aspects of trial design & execution. Patient-centric drug development also offers a huge opportunity to define meaningful outcomes from the patient perspective, as a way to ensure the needs and priorities of patient populations are reflected in research. Although efforts are made to control risks to clinical trial participants, some risk may be unavoidable because of the uncertainty inherent in clinical research involving new medical products. It's important, therefore, that people make their decision to participate in a clinical trial only after they have a full understanding of the entire process and the risks that may be involved.
Clinical Trials Conferences | Clinical Research Conferences | Clinical Reseach meetings
Relevant Conferences:
International Conference On Clinical Trials and Clinical Research, Toronto, Canada, September 24-26, 2018. Clinical Trial Supply Chain Strategy Meeting Europe 2018, 27 Sep 2018, Switzerland. Clinical Operations Strategy Meeting APAC 2018, 09 Oct 2018, Singapore. SCOE — Summit for Clinical Trials Operations Executives Europe, Oct 2018 Barcelona, Spain.
Track 11 :Research and Trials on Oncology and AIDS
HIV clinical trials are research studies that look at new ways to prevent, detect, or treat HIV/AIDS. Clinical trials are the fastest way to determine if new medical approaches to HIV/AIDS are safe and effective in people. clinical trials under way include studies of new HIV medicines, studies of vaccines to prevent or treat HIV, and studies of medicines to treat infections related to HIV.
There are several types of cancer clinical trials, including treatment trials, prevention trials, screening trials, and supportive and palliative care trials. Each type of trial is designed to answer different research questions and will help researchers learn things that will help people in the future.
Clinical Trials Societies | Clinical Research Societies | Clinical Trials and Research Associations
Relevant Conferences:
Basket and Umbrella Trials for Clinical Oncology, October 17-18, 2018, Philadelphia, PA. 7th Annual Publication and Clinical Trial Transparency, June 20-21, 2018, Amsterdam, The Netherlands. Direct-to-Patient Clinical Trials, August 9-10, 2018, Philadelphia, PA. 7th International Conference on Clinical Trials, September 24-26, 2018, Chicago, Illinois, USA.
Track 12 :Pharmacovigilance and Drug Safety
The pharmacovigilance is related to collection, detection, assessment, monitoring, and prevention of adverse effects with pharmaceutical products, and it is needed in different stages of product life cycle, and the safety surveillance and risk management. Information received from patients and healthcare providers via pharmacovigilance agreements, plays a critical role in providing the data necessary for Pharmacovigilance to take place, in order to market or to test a pharmaceutical product, adverse event data must be submitted to the local drug regulatory authority. Finally pharmacovigilance is concerned with identifying the hazards associated with pharmaceutical products and with minimizing the risk of any harm that may come to patients by safety surveillance and risk management
Clinical Trials Societies | Clinical Research Societies | Clinical Trials and Research Associations
Relevant Conferences:
Basket and Umbrella Trials for Clinical Oncology, October 17-18, 2018, Philadelphia, PA. 7th Annual Publication and Clinical Trial Transparency, June 20-21, 2018, Amsterdam, The Netherlands. Direct-to-Patient Clinical Trials, August 9-10, 2018, Philadelphia, PA. 7th International Conference on Clinical Trials, September 24-26, 2018, Chicago, Illinois, USA.
Track 13 :Bioinformatics in Clinical Research
Bioinformatics in Clinical Research the typically controversial ethical issues emerging from new situations and possibilities brought about by advances in medicine. It is also moral discernment as it relates to medical policy, practice, and research. Bioethicists are concerned with the ethical questions that arise in the relationships among life sciences, biotechnology, medicine, clinical research, and philosophy etc. One of the first areas addressed by modern bioethicists was that of human experimentation. The National Commission for the Protection of Human Subjects of Biomedical and Behavioural Research was initially established in 1974 to identify the basic ethical principles that should underlie the conduct of biomedical and behavioral research involving human subjects.
Clinical research ethics are the set of relevant ethics considered in the conduct of a clinical trial in the field of clinical research. It borrows from the broader fields of research ethics and medical ethics. Quality of clinical trials depends on data integrity and subject protection. Good Clinical Practice (GCP) is the universal ethical and scientific quality standard for conducting clinical trials. The GCP standard applies to all aspects of the clinical trial process.
Bioinformatics Conferences | Biostatistics Conferences | Clinical Trials Conferences
Relevant Conferences:
International Conference On Clinical Trials and Clinical Research, Toronto, Canada, September 24-26, 2018. Clinical Trial Supply Chain Strategy Meeting Europe 2018, 27 Sep 2018, Switzerland. Clinical Operations Strategy Meeting APAC 2018, 09 Oct 2018, Singapore. SCOE — Summit for Clinical Trials Operations Executives Europe, Oct 2018 Barcelona, Spain.
Track 14 :Clinical Trials on Different Diseases
Clinical Trials for different diseases and disorders are conducted for evaluating one or more interventions (for example, drugs, medical devices, approaches to surgery or radiation therapy) for treating a disease, syndrome, or condition and also finding ways to prevent the initial development or recurrence of a disease or condition. These can include medicines, vaccines, or lifestyle changes, among other approaches. Some examples of the diseases/disorders for which clinical trials conducting are Cardiovascular, Digestive system, Respiratory system diseases and other parasitic, viral, bacterial and fungal diseases. And Clinical Trials on behaviors, mental, sleep and eating disorders.
Rare Disease Conferences | Medicine Conferences | Medical Research Conferences
Relevant Conferences:
International Conference On Clinical Trials and Clinical Research, Toronto, Canada, September 24-26, 2018. Clinical Trial Supply Chain Strategy Meeting Europe 2018, 27 Sep 2018, Switzerland. Clinical Operations Strategy Meeting APAC 2018, 09 Oct 2018, Singapore. SCOE — Summit for Clinical Trials Operations Executives Europe, Oct 2018 Barcelona, Spain.
Track 15 :CRO (Contract Research Organization) Clinical Trials
CRO (Contract Research Organization) is an organization that provides support to the pharmaceutical, biotechnology, and medical device industries in the form of research services outsourced on a contract basis. Many CROs specifically provide clinical-study and clinical-trial support for drugs and/or medical devices. CROs range from large, international full-service organizations to small, niche specialty groups. A CRO may provide such services as biopharmaceutical development, biologic assay development, commercialization, preclinical research, clinical research, clinical trials management, and pharmacovigilance. CROs also support foundations, research institutions, and universities, in addition to government organizations.
Sponsorship: In the conduct of clinical trials, a sponsor is an individual (institution, company or organization) that takes the responsibility to initiate, manage or finance the clinical trial, but does not actually conduct the investigation. A sponsor-investigator, on the other hand, takes on the responsibility as a clinical study sponsor and also conducts or oversees the clinical trial. Thus, a sponsor-investigator must comply with the applicable regulatory requirements that pertain to both the sponsor and the investigator
Clinical Research Conferences | CRO Meetings | CRO Conferences
Relevant Conferences:
Basket and Umbrella Trials for Clinical Oncology, October 17-18, 2018, Philadelphia, PA. 7th Annual Publication and Clinical Trial Transparency, June 20-21, 2018, Amsterdam, The Netherlands. Direct-to-Patient Clinical Trials, August 9-10, 2018, Philadelphia, PA. 7th International Conference on Clinical Trials, September 24-26, 2018, Chicago, Illinois, USA.
Track 16 :Future Perspectives and Innovations in Clinical Studies
Clinical study design comprises the quantity of study volunteers, their segmentation based on varying factors, and their treatment throughout the clinical trial process. Study design is a key component of clinical trials, and the treatment of all patients directly impacts the statistical validity of data. Study group assignment has also been comprehensively improved in recent years. Researchers have found many benefits to randomized assignment versus observational assignment, based on characteristics like gender, age, race, etc. The randomized method has been found to yield more reliable results than observational study assignments.
In recent years, the use of Adaptive design methods in clinical research has become increasingly popular due to its flexibility and efficiency. Adaptive designs offer the potential to reduce study duration and patient exposure whilst maximizing the probability of a successful outcome. Another innovation in clinical trials is the Bucket design. Bucket trials are designed to utilize one particular drug and test that drug against a number of different diseases. The advantage of this approach is that patients with different diseases can be 'pooled' together under one larger trial instead of lots of smaller trials, thereby saving time and resource in a similar approach, and there are more innovations in clinical trials.
Clinical Trials Conferences | Clinical Research Conferences | Clinical Research Meetings
Relevant Conferences:
Basket and Umbrella Trials for Clinical Oncology, October 17-18, 2018, Philadelphia, PA. 7th Annual Publication and Clinical Trial Transparency, June 20-21, 2018, Amsterdam, The Netherlands. Direct-to-Patient Clinical Trials, August 9-10, 2018, Philadelphia, PA. 7th International Conference on Clinical Trials, September 24-26, 2018, Chicago, Illinois, USA.
Track 17 :In Silico Clinical Trials
Generally accepted principles suggest that patient involvement should extend well beyond consideration as research subjects. Patients are key stakeholders in all aspects of trial design & execution. Patient-centric drug development also offers a huge opportunity to define meaningful outcomes from the patient perspective, as a way to ensure the needs and priorities of patient populations are reflected in research. Although efforts are made to control risks to clinical trial participants, some risk may be unavoidable because of the uncertainty inherent in clinical research involving new medical products. It's important, therefore, that people make their decision to participate in a clinical trial only after they have a full understanding of the entire process and the risks that may be involved.
Clinical Trials Conferences | Clinical Research Conferences | Healthcare Conferences
Relevant Conferences:
International Conference On Clinical Trials and Clinical Research, Toronto, Canada, September 24-26, 2018. Clinical Trial Supply Chain Strategy Meeting Europe 2018, 27 Sep 2018, Switzerland. Clinical Operations Strategy Meeting APAC 2018, 09 Oct 2018, Singapore. SCOE — Summit for Clinical Trials Operations Executives Europe, Oct 2018 Barcelona, Spain.
Track 18 : Outsourcing and Collaborative Research in Clinical Trials
Outsourcing and Collaborative Research in Clinical Trialsbrings together CROs, sites, sponsors and vendors to collaborate on best practices for ensuring accurate and effective budgeting and forecasting in clinical trials. With growing complexities and shrinking tolerance for variance between forecasted and actual budget, it’s critical that internal and external teams work together to tackle the challenges and establish efficient processes. Join your trial counterparts in May to bridge the gap between finance and operations and address each player’s role in managing deviations including timeline delays, changes in study design, outsourcing and more.
Clinical Outsourcing | Clinical Research Conferences | Clinical Trials Conferences
Relevant Conferences:
Basket and Umbrella Trials for Clinical Oncology, October 17-18, 2018, Philadelphia, PA. 7th Annual Publication and Clinical Trial Transparency, June 20-21, 2018, Amsterdam, The Netherlands. Direct-to-Patient Clinical Trials, August 9-10, 2018, Philadelphia, PA. 7th International Conference on Clinical Trials, September 24-26, 2018, Chicago, Illinois, USA.
Track 19 :Gastrointestinal-oncology
Gastrointestinal oncology refers to malignant conditions of the gastrointestinal tract (GI tract) and accessory organs of digestion, including the oesophagus, stomach, biliary system, pancreas, small and large intestine, rectum and anus. The symptoms relate to the organ affected and can include obstruction which leads to difficulty swallowing or defecating, abnormal bleeding. The treatment requires endoscopy, followed by biopsy of suspicious tissue. The treatment depends on the location of the tumor, as well as the type of cancer cell and whether it has invaded other tissues or spread elsewhere. These factors also determine the prognosis.
Bioethics Conferences | Ethics Conferences | Clinical Trials Ethics
Relevant Conferences:
International Conference On Clinical Trials and Clinical Research, Toronto, Canada, September 24-26, 2018. Clinical Trial Supply Chain Strategy Meeting Europe 2018, 27 Sep 2018, Switzerland. Clinical Operations Strategy Meeting APAC 2018, 09 Oct 2018, Singapore. SCOE — Summit for Clinical Trials Operations Executives Europe, Oct 2018 Barcelona, Spain.
Track 20 :Entrepreneurs Investment Meet
Since the Designing and development of a pharmacological product is a process, single organizations cannot take up the cost of research involved in it. Entrepreneurs willing to invest in the Pharma sectors can meet together and have an idea about the Pharma product, it’s effectiveness, it’s market potential and scope. Since the Pharma sector is booming in the recent market scenario, investors can have a great share of profits.
Clinical Trials Societies | Clinical Research Societies | Clinical Trials and Research Associations
Relevant Conferences:
International Conference On Clinical Trials and Clinical Research, Toronto, Canada, September 24-26, 2018. Clinical Trial Supply Chain Strategy Meeting Europe 2018, 27 Sep 2018, Switzerland. Clinical Operations Strategy Meeting APAC 2018, 09 Oct 2018, Singapore. SCOE — Summit for Clinical Trials Operations Executives Europe, Oct 2018 Barcelona, Spain.
Importance & Scope:
Clinical trials are an important step in discovering new treatments and other diseases as well as new ways to detect, diagnose, and reduce the risk of disease. Clinical trials show researchers what does and doesn’t work in people. Clinical trials also help researchers and doctors decide if the side effects of a new treatment are acceptable when weighed against the benefits offered by the new treatment.
Clinical trials, unlike observational studies, contain an intervention and determines the safety, efficacy and dosing of a drug. Clinical trials may be randomized in which groups of participants are randomly selected to either receive the standard of care or the drug under study. Clinical trials are generally blinded, meaning the participant’s physician and the study team is unaware of the treatment that the participant is receiving. This protects the integrity of the data by eliminating bias or causing a “placebo effect.”
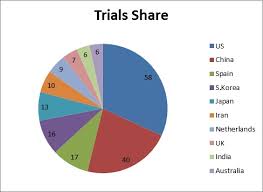
Figure 1: Trials Share
Future prospects of Clinical Trials:
- Data Integration and Visualization
- Personalized Medicine
- Individualized Data
- Combination Trials
- Mobile Technology
Why Australia:
Australia’s strong economy, skilled and multilingual workforce, diverse population and robust system for the protection of intellectual property make it an ideal location for conducting clinical trials. Australia is regarded as a global leader in conducting clinical trials and is home to some of the world’s esteemed scientists, physicians and healthcare professionals. It boasts world-class medical research and healthcare infrastructure. The country has an efficient regulatory system, including a rapid clinical trials approval system.
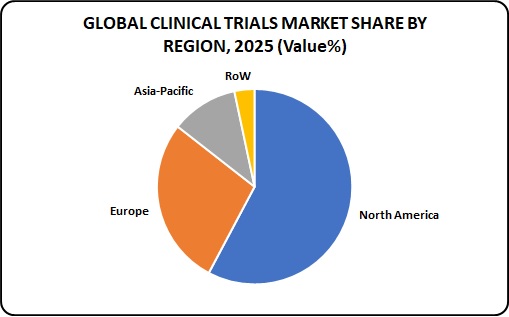
Figure 2: Global Clinical Trials Market
Conference Highlights:
- Clinical Research & Clinical Trials: Academic Perspective
- Clinical Trials Conducts
- Clinical Data Management
- Pharmacogenomics
- Pharmacoepidemiology
- Patient-Centric Clinical Trials
- Pharmacovigilance & Drug Safety
- Bioinformatics in clinical research
- Post marketing surveillance
- Clinical Trials on different Diseases
- CRO (Contract Research Organization) clinical trials
- Drug Discovery and Development
- Future Perspectives and Innovations in Clinical Studies
- Clinical and medical case reports
- Globalization of clinical trials
- In silico clinical trials
- Outsourcing and Collaborative research in clinical trials
- Ethics in clinical trials
- Nano technology in clinical trials
- Entrepreneur’s investment meet
Target Audience:
CRO’s, Pharmacy professionals, Association chiefs and Pharma Business people. Professors, Students and to provide an international forum for the spread of original research results, new ideas and practical development experiences which concentrate on both theory and practices, CEO's and Scientists, R & D Professionals
- Industry 50%
- Researchers 20%
- Academia 20%
- Others 10%
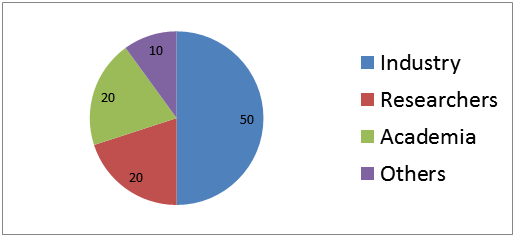
Figure 3: Target Audience Analysis
Market Growth of Clinical Trials:
In 2015, 1,360 clinical trials were started in Australia, including 473 industry sponsored trials [i]. Australian clinical trials are growing at a fast rate, outpacing the U.S., the UK and the global average by roughly five-per-cent per year. Australia invested $1.1 billion in gross expenditure on on-going trials in 2015, which included $930 million from industry sponsors with the exciting potential to surpass $2 billion in the next 10 years.
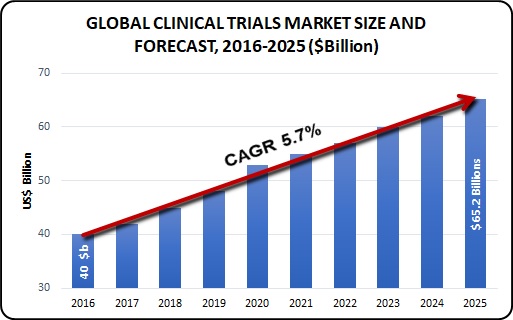
Figure 4: Market Size and Forecast
Global pharmaceutical market forecast, 2010-2018:
The worldwide clinical trials advertise is required to achieve USD 65.2 billion by 2025, as indicated by another report by Grand View Research, Inc. Key drivers affecting the market development are globalization of clinical trials, improvement of new medications, for example, customized drug, enlarging advancement in innovation, and boosting interest for CROs to lead clinical trials. Globalization of clinical trials has prompted increment in interest in new item improvement in rising nations in this manner, positively affecting generally speaking business sector. The accessibility of the immense range of administrations from sedate disclosure to post-showcasing reconnaissance has additionally improved the life for moderate size and little scale pharmaceutical and biotechnological associations by giving them the choice to outsource what they believe is past their center aptitude.
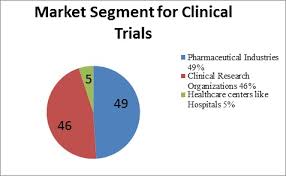
Figure 5: Market Segment for Clinical Trials
Conference Highlights
- Clinical Research & Clinical Trials: Academic Perspective
- Clinical Trials Conducts
- Clinical Data Management
- Pharmacogenomics
- Pharmacoepidemiology
- Patient-Centric Clinical Trials
- Pharmacovigilance and Drug Safety
- Bioinformatics in Clinical Research
- Clinical Trials on Different Diseases
- CRO (Contract Research Organization) Clinical Trials
- Drug Discovery and Development
- Future Perspectives and Innovations in Clinical Studies
- Clinical and Medical Case Reports
- Globalization of Clinical Trials
- In Silico Clinical Trials
- Outsourcing and Collaborative Research in Clinical Trials
- Nano Technology in Clinical Trials
- Entrepreneurs Investment Meet
- Gastrointestinal oncology
- Research and Trials on Oncology and AIDS
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | November 19-20, 2019 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | |||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Clinical Trials
- Journal of Clinical Research & Bioethics
- Journal of Clinical Case Reports
Abstracts will be provided with Digital Object Identifier by

















